C. difficile Infection Management
Clostridium difficile, a major cause of healthcare-associated infections (HAI), is classified as an “urgent threat” in the CDC’s latest report on Antibiotic Resistance Threats¹. Its increasing prevalence and high levels of morbidity and mortality are serious concerns on both human health and economic fronts. C. difficile infection management involves a full-circle range of measures covering prevention, diagnosis, therapies, surveillance, outbreak management and epidemiology. With its global solution, bioMérieux is your partner in fighting C. difficile.
További tájékoztatást szeretne?
Situation: C. difficile “urgent” threat today
|
Clostridium difficile infections Estimated Infections annually: Estimated economic burden: 1. Antibiotic Resistance Threats in the United States, CDC 2013 |
Once upon a time, Clostridium difficile was considered little more than a clinical nuisance. Today, C. difficile is one of our most challenging healthcare-associated infections (HAI), accounting for more than 60% of all cases of healthcare-associated diarrhea². In most cases, C. difficile is contracted in the healthcare setting and is connected to antibiotic treatment. C. difficile infection incurs costly treatment, patient isolation and longer hospital stays. In addition to the impact on patients themselves, this means high economic burden on hospitals and healthcare systems. C. difficile is at historically high levels worldwide, according to the Centers for Disease Control. Hyper-virulent strains are emerging, and we are seeing severe outbreaks, and morbidity and mortality rising at alarming rates¹.
There is also evidence that C. difficile transmission is spreading out of the healthcare setting into the community³. In fact, the CDC classified C. difficile in 2013 as an “urgent threat”, meaning “an immediate public health threat that requires urgent and aggressive action”1.
1. Centers for Disease Control and Prevention : ANTIBIOTIC RESISTANCE THREATS in the United States, 2013
2. Point Prevalence Survey of Healthcare Associated infections and Antimicrobial use in European acute care hospitals,
2011-2012 – ECDC surveillance report, July 2013
3. Wilcox et al. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother 2008;
62:388-96.
Challenge: Managing C. difficile from prevention to epidemiological trending
Fighting today’s complex healthcare challenges like C. difficile requires a multi-faceted approach. Healthcare institutions, medical professionals, microbiologists, patients – we are all involved.
At bioMérieux, we are your dedicated partners, bringing our longstanding, recognized expertise in microbiology, and our innovative laboratory solutions to support your actions.
C. difficile infection management starts with prevention and moves along a continuum including diagnosis, treatment/surveillance, outbreak management and epidemiological trending. The microbiology lab is challenged to provide fast, accurate diagnostic and surveillance answers as well as reliable records for trending. There are a number of needs to ensure better responses to the challenges at every stage of C. difficile infection management:
Prevention/infection control needs:
- Effective hygiene and cleansing procedures
- Antimicrobial stewardship: Adapted therapies to be implemented at the right time
- Education and involvement of healthcare staff (e.g. correct hygiene/hand-washing, antibiotic use, etc.)
- Effective, environmental control tests
Diagnosis needs:
- Rapid, accurate, easy and cost-effective diagnostic tools including instruments and reagents
- Good testing algorithm guidelines
- Increased awareness
Outbreak management needs:
- Fast and easy genotyping tools for C. difficile strain genotyping and outbreak management
- Reliable data management to optimize information flow
Epidemiology needs:
- Accurate, complete and easy-to-access data collection and reporting to track trends
- Good information flow across regions
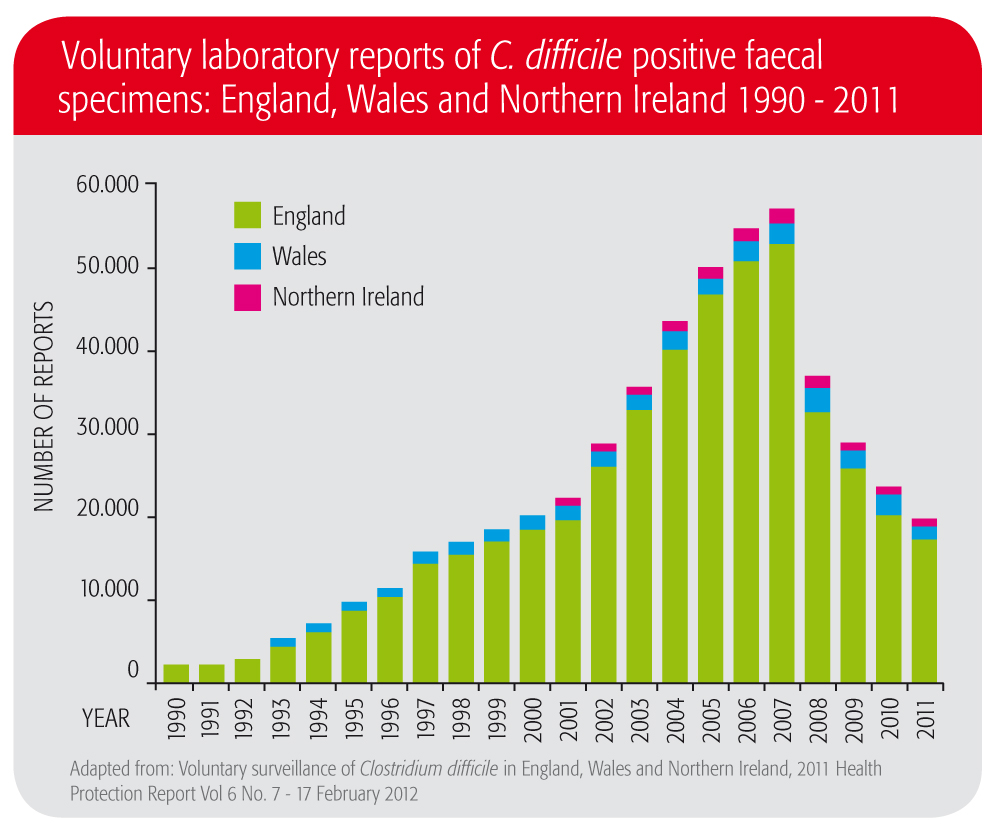
The good news – it can be doneIn the UK, C. difficile infection rates have been decreasing since 2007.
|
Solution: Accurate, flexible, accessible ways to meet the C. difficile challenge
bioMérieux has been on the frontlines of the fight against C. difficile since the beginning. Not only do we listen to your needs, we take a global approach to meeting them, with instruments and reagents needed to support investigation and decision-making for C. difficile infection management. With a high level of integrated automation, our instruments are developed to offer ease-of-use, reliability and traceability. Plus, our range is flexible and accessible – to suit different lab types and sizes, and different management needs.
| Requirement |
bioMérieux solution |
|---|---|
| Infection control | Count-Tact® surface testing range |
| Screening | VIDAS® GDH |
| Toxin detection | VIDAS® C. difficile Toxin A&B |
| Culture | chromID® C. difficile agar Clostridium difficile agar |
| Identification | VITEK® 2 ANC card API® 20A rapid ID 32 A |
| Susceptibility testing | ATB™ ANA2 Etest® |
| Strain typing | DiversiLab® C. difficile |
“ VIDAS® Clostridium difficile GDH is an automated test,
allowing easier interpretation and traceability of results. ”
Comparison of the VIDAS® C. difficile GDH and the GDH component of the C. diff
Quik Chek Complete for detection of Clostridium difficile in stools.
C. Eckert, et. al. (Paris, FR)ECSMID: 29.04.2013
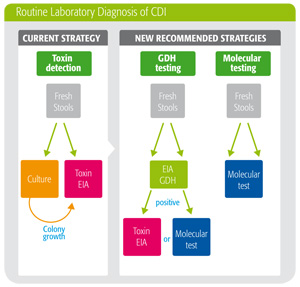
Solution: New testing algorithm guidelines
New C. difficile testing algorithm guidelines recommend the use of a two-step protocol starting with a GDH enzyme immunoassay (EIA) followed by a sensitive toxin EIA to confirm positive results, and finally a culture test to establish sensitivity. While this testing protocol provides results similar to the “gold standard” culture + toxin test, it cuts costs and saves time.
|
Selected guidelines A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations. Guide to Preventing Clostridium difficile Infections. Updated guidance on the diagnosis and reporting of Clostridium difficile. |
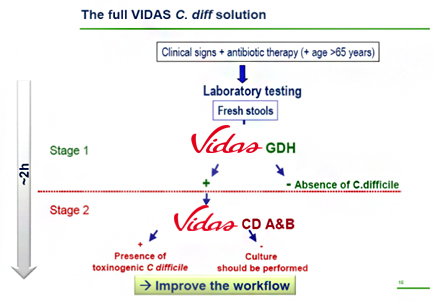
VIDAS® GDH & VIDAS® CD A&B
Effective implementation of the new two-step guidelines, requires state-of-the-art instruments and reagents. bioMérieux’s VIDAS® C. difficile solution, combining VIDAS® GDH and VIDAS® CD A&B, offers a smart solution. Here’s how:
- Automated and easy-to-use:
- the VIDAS® immunoanalyzer family is recognized worldwide for its flexibility, ease-of-use and seamless fit into lab workflow
- Cost-effective:
- Reduces unnecessary tests
- Single-test format adapted to low or high volume testing for sporadic or epidemic outbreaks
- Tracking epidemiological trends: keep record of all processed specimens
chromID® C. difficile
For culture testing, bioMérieux’s chromID® C. difficile offers clear identification in just 24 hours1,2, up to 30% more sensitive than conventional media² Using a patented chromogenic substrate and mixture of antibiotics, the medium is highly selective. Results are extremely easy to read, with black/dark grey colonies contrasting clearly on a pale agar.
-
Boseiwaqa LV, et. al. Comparison of ChromID C. difficile agar and cycloserine-cefoxitin-fructose agar for the recovery of Clostridium difficile. Pathology. 2013 Aug;45(5):495-500. doi: 10.1097/PAT.0b013e3283632680.
-
Perry D. J. et al. Evaluation of a Chromogenic Culture Medium for isolation of Clostridium difficile within 24 hours. Journal of Clinical Microbiology– 2010; 48:3852-3858.
DiversiLab®
The DiversiLab® system provides strain typing results in approximately 4 hours. Using rep-PCR* technology, the automated platform provides standardized, reproducible DNA fingerprinting for C. difficile samples. The system also enables analysis of other bacterial and fungal samples for complete isolate characterization.
rep-PCR: amplification of non-coding repetitive sequences interspersed throughout the bacterial genome using Polymerase Chain Reaction (PCR). The rep-PCR technology and rep-PCR primers are covered by U.S. patents (5,691,136 and 5,523,217) and by international patents for Canada and Europe.
NOTE: DiversiLab is not for diagnostic use.
C. difficile is an urgent public health issue – we are dedicated to helping manage it
|
bioMérieux knows labs With more than 50 years’ experience working with microbiology labs, we’ve been at your side throughout the evolution of challenges facing labs around the world – including the emergence of the C. difficile threat. |
Advances are needed at all levels (medical, scientific, public policy, etc.) to ensure continual improvement in the management of C. difficile infections. In addition to our comprehensive approach to C. difficile diagnostics, bioMérieux contributes through scientific and educational initiatives like these:
- The World Forum on Healthcare Associated Infection and Antimicrobial Resistance, organized by bioMérieux, brings together over 70 international leaders in the field to discuss and drive new strategies to respond to HAI/Resistance challenges. The 4th edition of this unique event was held in June, 2013.
- Our activities for Antibiotic Awareness Week in November, 2013 included the release of a Practical Guide to Antimicrobial Stewardship in Hospitals.
- Our informational publications include Clostridium Difficile Infections from diagnosis to Outbreak Management and A Practical Guide for the Prevention of Healthcare Associated Infections
- Our Be S.M.A.R.T. with Resistance™ solutions to manage the antimicrobial resistance threat, including information, education and events.
- We develop educational partnerships with leading associations like the French National Observatory for Epidemiology of Bacterial Resistance to Antimicrobials (ONERBA).
- We support young microbiologists through grants and awards – knowing that tomorrow’s leaders in the field will help us improve responses to our greatest healthcare challenges.